Plants and Polarization
Indubitably, photovoltaic
cells will have to improve in design and efficiency to compete with other sources.
The chloroplasts in plants, nature’s own solar cells, could serve as a guide
for human-made ones. Artificially-grown chlorophyll could function as an n-type
semiconductor (see PRESENT), since it releases excited electrons when exposed
by light. In nature, when sunlight contacts each assembly of some 300 chlorophyll
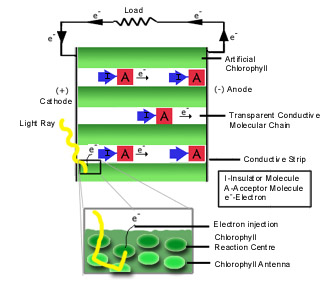
molecules, only a single pair of pigment molecules, called the “reaction centre,”
donates excited electrons to an acceptor. The other molecules only transfer
energy to the reaction centre, and are called “antennae.”6 In the
future, these specified molecules will be modified to increase efficiency significantly.
As well, in a chlorophyll-photovoltaic cell (nicknamed the “chlorovoltaic cell,” or “CVC”) it will be useful to employ multiple layers of synthetic chlorophyll, each specializing in absorbing certain wavelengths of light. For instance, layers of chlorophyll-a and chlorophyll-b (both synthesized from plants) will specialize in blue-violet-red and orange-blue wavelengths respectively. New layers can be engineered using developed technologies such that they are able to absorb the energy from ultraviolet, yellow-green, and infrared light. With each layer parallel to one another, incident light will be used to its maximum capability.
The electrons released by chlorophyll can be harnessed using conductive molecular chains, essentially the p-type semiconductor (Fig. 1). Long-chain polymers that accept electrons and transport them may be used. To increase efficiency, the electron flow in these chains will be unidirectional with the help of special Iad structures (see INSIGHTS). Via the chains electrons will be transported to the conduction strip (possibly made of tin oxide) into an external circuit connected to the load. Another strip (possibly platinum) will reintroduced them into the chlorophyll, and the cycle will repeat.
In present-day conventional solar cells, maximum efficiencies of only 33% can be achieved due to limitations by the thermodynamics law stating that entropy increases5. Photons from sunlight incident on pv cells have randomly-oriented electric vectors that are absorbed to produce excited electrons moving in random directions, thus exhibiting high entropy and useful energy loss into heat. In the CVC, efficiency will be greatly increased by light-polarizing each layer such that randomly-oriented photons are aligned into organized vectors. As light passes though this polarized film, only photons striking along the direction of its “stretch axis” will be absorbed, and excited electrons will travel in the same direction; the rest of the light will pass through to the next layer. By positioning two polarized layers with their stretch axes mutually perpendicular, each layer will absorb roughly half the incident light. Such a concept for solar cells is currently being investigated, though improvement is still needed26. Efficiency of such devices is predicted to be around a remarkable 72%25.
The artificial chlorophyll in CVCs will be chemically placed unto a transparent sheet, such as Mylar™ film, in a linear wire-grid pattern for polarization purposes. The conductive molecular chains with the Iads will likewise be placed, in the spaces between wires. For protection, clear plastic or polymer material will be laminated as a cover. CVC layers will be extremely thin (less than a micron) and versatile, since only a fine film is needed to collect and convert light, the cell should be able to cover various shapes, and less material is used. Current thin-film techniques of stretching and flattening can be applied.